Mercury in flue gas (HgT、Hg0、Hg2+) The technology of continuous online monitoring and analysis application mainly includes the technology of continuous online analysis of mercury in flue gas, the technology of reducing and transforming elemental mercury from oxidized state, the technology of preparing standard materials of gaseous mercury, the technology of enriching and pyrolysis of gaseous mercury in flue gas, and the technology of dynamic labeling recovery calibration, etc., which are briefly introduced below.

1. Mercury Analysis Instrument Technology
- Cold vapor atomic absorption spectroscopy (CVAAS)
The CVAAS measurement used for mercury analysis is based on the selective absorption of 253.7nm-wavelength light by mercury atoms. Within a certain concentration range, absorbance is proportional to mercury concentration. After digestion of the sample, various valence states of mercury are converted into divalent mercury, which is then reduced to elemental mercury using stannous chloride for measurement.
- Semian modulated atomic absorption spectroscopy (ZAAS)
The ZAAS measurement used for mercury analysis is based on Zeeman cold atom absorption technology. The 253.7nm resonance line of mercury is split into three Zeeman components (π, σ-, σ+). When mercury vapor is present in the sample cell, the difference in σ component light intensity increases with the increase in mercury vapor concentration. The difference in σ component light intensity can be directly subtracted from the background interference. Zeeman modulated atomic absorption spectroscopy technology has been matured for online continuous measurement systems for flue gas mercury analysis.
- Cold vapor atomic fluorescence (CVAFS)
The CVAFS measurement used for mercury analysis is based on the combination of atomic fluorescence analysis technology and mercury vapor generation sampling at room temperature. It converts mercury compounds in the sample to elemental gaseous mercury atoms using a strong reducing agent. The sample is then exposed to excitation light with a wavelength of 253.7nm emitted by a low-pressure mercury lamp. The ground-state mercury atoms are excited to higher energy states, and when they return to the ground state, they emit resonance fluorescence. The intensity of this fluorescence is measured by a photomultiplier tube. Since only mercury atoms fluoresce, this method enhances the selectivity of mercury detection.
- Atomic emission spectroscopy (AES)
Atomic emission spectroscopy for mercury measurement is based on the emission of 253.7nm characteristic wavelengths by mercury atoms caused by plasma. This method can measure mercury in any form. Since each component is dissociated into elemental forms by the plasma source before the detector, it is less susceptible to interference from other components. Atomic emission spectroscopy can also measure various heavy metal elements.
- Ultraviolet differential absorption spectroscopy (UV-DOAS)
Mercury detection using ultraviolet differential absorption spectroscopy is based on the strong absorption line of elemental mercury at 253.7nm. UV-DOAS (Ultraviolet Differential Optical Absorption Spectroscopy) uses a molecular narrowband absorption technique that splits the absorption cross-section into two components: one that changes quickly with wavelength and another that changes more gradually. By focusing on the component that changes rapidly with wavelength, UV-DOAS effectively minimizes the interference from Rayleigh and Mie scattering, as well as the attenuation of light intensity caused by gases and particles in the smoke. This technique is highly effective for detecting trace gases in the atmosphere, including the monitoring of small amounts of mercury.
In addition, xray fluorescence (XRF) spectroscopy is also applicable to the monitoring of trace mercury in the atmosphere.
2. Reduction of Gaseous Oxidized Mercury to Elemental Mercury Technology
Measure Hg in flue gas2+In this case, Hg must be added2+turn into Hg0The converter technology is mainly divided into three types.
- Wet chemical reduction method
Wet chemical reduction is used to reduce Hg under acidic medium conditions2+turn into Hg0:
HgCl2+ SnCl2 →Hg+ SnCl 4
To measure samples containing low concentrations of mercury, it is typically used in conjunction with enrichment methods that form gold amalgam. Gaseous mercury is obtained through wet chemical reduction, allowing it to be separated from other pollutants in the flue gas, thus avoiding contamination of analytical equipment and cross-interference from other components. Additionally, the detection limit can be adjusted by varying the enrichment time.
- Thermal catalytic reduction method
Heated mercury-containing sample gas to 200~400℃, and Hg in flue gas will be removed under the action of catalyst2+turn into Hg0This method is relatively simple and easy to implement, but there are some problems such as the influence of conversion efficiency on measurement results and the operating life of catalyst.
- High temperature oxidation reduction method
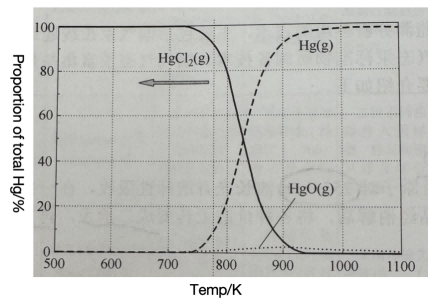
Heat the sample to 800~1000℃ to make Hg2+turn into Hg0The relationship between mercury valence state and temperature is shown in the above figure. As can be seen from the figure, Hg0More than 96%.
The above methods are compared as follows: Wet chemical reduction is a classic standard method used in the laboratory, but it consumes and treats chemical reagents continuously, and the process is complicated. The advantage of thermal catalytic reduction is that it is simple and easy to perform, but the agent will be affected by acidic substances such as SO in the flue gas2, HCI and other corrosion reduces the service life. High temperature oxidation reduction method requires high equipment manufacturing and material performance, but the long-term conversion efficiency is high and stable, no reagent and material consumption, so the method is reliable in the long term.
At present, high temperature oxidation reduction conversion technology has been widely used in the online analysis system of flue gas mercury. At high temperature, pyrolysis of mercury compounds and Hg are desorbed2+turn into Hg0Then enter the measurement absorption cell for quantitative measurement.
3. Preparation Technology of Gaseous Mercury Standard Material
Hg is used for mercury meter calibration0Generator/calibrator, for the system calibration of Hg-CEMS, use HgCl2Calibration gas generator/calibrator; alternatively, qualified cylinders of Hg calibration gas can be used, or the system calibration of Hg-CEMS can be performed using the permeation method. Different analytical techniques can be calibrated using different instruments.
At present, there are two main methods for preparing mercury standard materials built-in and external to the system.
One type is a mercury standard gas generator designed based on the permeation tube method. It includes a mercury source, a constant temperature condenser, and a mixing chamber connected in sequence by carrier gas tubing. The constant temperature condenser consists of a water bath controlled by a constant temperature controller, several series-connected gas washing bottles, and a gas temperature sensor. Mercury vapor produced by the mercury source enters the gas washing bottles through the carrier gas tubing, condensing into mercury saturated vapor at a certain temperature. This mercury saturated vapor then enters the mixing chamber to mix with diluent gas in proportion to form the mercury standard gas.
Another mercury standard gas generator is controlled by a mass flow meter to control the ratio of mercury chloride standard solution and dilute gas, and a vaporizer produces high temperature mercury chloride standard gas of certain concentration, which is delivered to the probe filter through a high temperature heating gas path, and finally enters the high temperature mercury reduction chamber to be converted into Hg0Perform instrument calibration. If the analyzer is inside the Hg with a permeable tube0The generator is used simultaneously. The system can automatically calibrate the range through electronic control, so that the zero point and range can be calibrated without introducing standard gas. The reduction of mercury chloride to Hg is monitored by the difference between the results of two different standard gas sources0The conversion efficiency.
In order to ensure the long-term reliability and authenticity of the measurement results, the automatic calibration device of the standard gas chamber (or standard filter) can be built into the measuring instrument, which also achieves the purpose of controlling the drift of the instrument and ensuring the accuracy of the measurement results.
CEMS LX-4000-Hg Stack Mercury Continuous Monitoring System

ESEGAS has harnessed CV AFS technology to create an advanced continuous monitoring system for mercury emissions, known as the Hg stack mercury monitoring system. This innovative system is designed to detect the real-time concentration of mercury being emitted and to calculate its cumulative emission rate accurately.
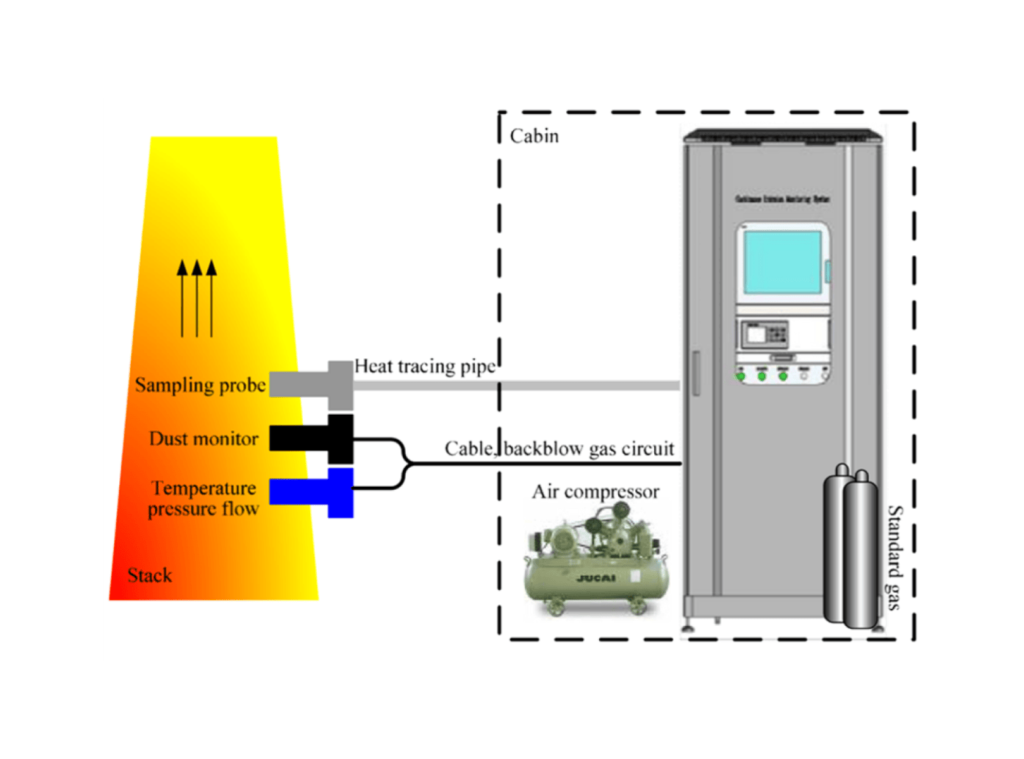
CEMS LX-4000-Hg stack mercury continuous monitoring system utilizes Cold Vapor Atomic Fluorescence Spectrophotometer technology to effectively measure the real-time concentration of mercury emissions and track their cumulative emission rate. This advanced system can monitor various forms of mercury, including gaseous elemental mercury, ionic mercury, and total gaseous mercury, providing a comprehensive analysis of mercury levels in emission.